Diabetes Care App for Type 2 Diabetes
Research Study Enrolling in West Palm Beach FL
![]()
The diabetes care app is a smartphone app available on iOS and Android platforms intended to provide relevant education content and tracking capabilities for people with diabetes, specifically focusing on those with type 2 diabetes.
To learn more about this study, call Metabolic Research Institute Inc. (561) 802-3060, ext. 8036 or visit:
https://www.metabolic-institute.com/BD
ABOUT METABOLIC RESEARCH INSTITUTE, INC. (“MRI”)
Clinical studies are an integral part of clinical research and are at the heart of all advances in modern medicine. Our mission at the Metabolic Research Institute is to provide our patients and sponsors with the highest quality of diabetes clinical research available in today’s medical professional environment. We are committed to delivering efficient and timely research data, while focusing on strict adherence to protocol guidelines.
Metabolic Research Institute, Inc. (“MRI”), is a private Clinical Research Company located at 1515 North Flagler Drive, Suite 440, West Palm Beach Florida 33401. Unless otherwise noted, all study-related appointments for qualified clinical participants in any MRI research trial will take place at this address
Type 1 Diabetes Clinical Study
Type 1 Diabetes Clinical Study enrolling now in West Palm Beach FL
Qualified candidates will be:
• Men and women 18 to 80 years of age
• Diagnosed with Type 1 Diabetes for at least 1 year
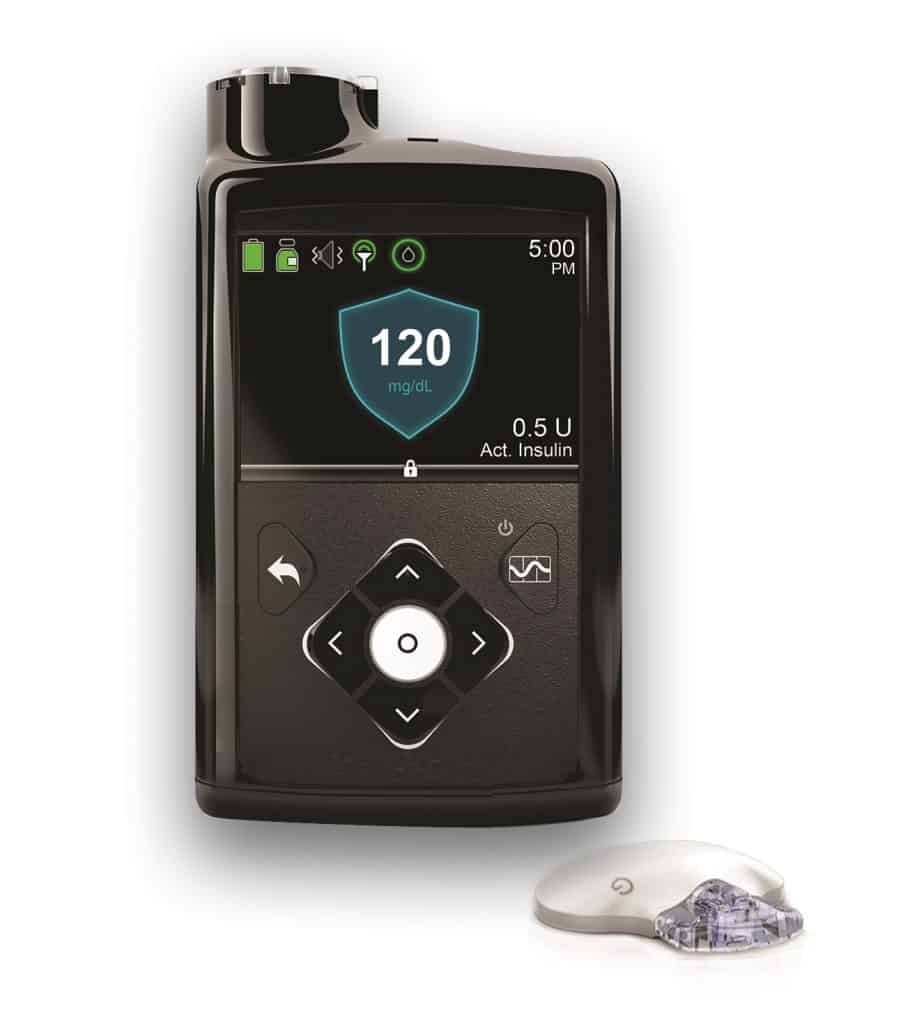
• Currently using the Medtronic Minimed 670G Insulin Pump
• Willing to wear each Extended Wear Infusion Set for at least 174 hours (7days & 6 hours) or until infusion set failure
Additional eligibility criteria also apply.
Qualified participants will receive all study-related medications, laboratory tests and study-related supplies at no cost. You may also be reimbursed for your time and travel.
The Extended Wear Infusion Set (EWIS) is an investigational study. Its purpose is to collect data to support the safety and effectiveness of wearing the EWIS infusion set for 6 to 7 days. This study will last approximately 12 to 16 weeks, and will consist of 5 on-site and 2 telephone visits.
Important Safety Information for MiniMed™ 670G System: The MiniMed™ 670G is for type 1 ages 7 and over. Prescription required. Individual results may vary. WARNING: May not be safe under age 7 or using less than 8 units/day.
For more information, please call: (561) 802-3060, or visit: https: www.metabolic-institute.com/EWIS
Metabolic Research Institute Inc.
Barry S. Horowitz, MD, FACE – Principal Investigator
1515 N. Flagler Drive, Suite 440
West Palm Beach, FL 33401
Study Sponsor: Medtronic Diabetes 18000 Devonshire Street Northridge, CA 91325
Insulin Affordability
Novo Nordisk’s new insulin affordability offerings now available in the US
• My$99Insulin: All patients can purchase up to three vials or two packs of FlexPen®/FlexTouch®/Penfill® pens of any combination of insulins from Novo Nordisk Inc. for $99
• Follow-on brand insulins: Authorized generics of NovoLog® and NovoLog Mix® made by new Novo Nordisk A/S US company, Novo Nordisk Pharma, Inc., available at pharmacies within 1-3 business days at 50 percent off the list price
• Immediate Supply: New, immediate, one-time insulin supply option available for people facing an acute need when more time is needed to identify a long-term sustainable solution
Visit NovoCare.com for more information on new and existing programs for people with and without insurance
PLAINSBORO, N.J., January 2, 2020 /PRNewswire/ – Today, Novo Nordisk launched the My$99Insulin Program, follow-on brands of insulins, and an Immediate Supply option, expanding its insulin affordability offerings to help people with diabetes who need alternative solutions. These options, along with information on the existing patient assistance program, copay cards, and where to find $25/vial human insulin, are now available online at NovoCare.com or by calling 1.844.NOVO4ME (1.844.668.6463).
“We know some people still struggle to afford their insulin and we want to help. We’ve talked to people, including those who have been critical of us, and it’s clear there is no one solution that will work for everyone and people need options,” said Doug Langa, executive vice president, North America Operations and president of Novo Nordisk Inc. “That’s why today, we have made available additional options recognizing the different situations that make insulin unaffordable or inaccessible. With these programs, in conjunction with NNI’s investment in rebates to ensure our medicines are on formularies, we are continuing to take action to help people with diabetes afford their insulin as we work toward much needed longer-term change.”